What Are Amines?
Amines are organic chemical compounds that contain nitrogen as their main element. They are formed when one or more hydrogen atoms in ammonia (NH₃) are replaced by carbon-based groups such as alkyl or aryl groups. Because of this structure, amines are closely related to ammonia but show different chemical behavior.
In simple words, amines are nitrogen-containing organic compounds that are widely found in nature and used in many industrial processes. They can exist as gases, liquids, or solids, depending on their molecular size. Many amines have a strong smell, similar to fish or ammonia.
Amines are a very important part of organic chemistry because they act as building blocks for many complex chemicals. From medicines to dyes and from plastics to agricultural chemicals, amines play a key role in modern life.
Why Amines Are Important in Chemistry and Industry?
Amines are important because they are highly reactive and versatile. In chemistry, they are used to make a wide range of compounds such as drugs, polymers, pesticides, and dyes. Their ability to react with acids and other chemicals makes them useful in many chemical reactions.
In industry, amines are used as:
- Raw materials for pharmaceuticals
- Gas treatment chemicals to remove CO₂ and H₂S
- Intermediates in plastic and rubber manufacturing
- Additives in paints, coatings, and detergents
Their basic nature helps in neutralization processes and chemical synthesis, which is why industries depend heavily on them.
Real-Life Relevance of Amines
Amines are present in everyday life more than we realize. Many medicines, including painkillers and antibiotics, contain amine groups. Proteins and amino acids, which are essential for the human body, are also based on amine chemistry.
They are used in:
- Fertilizers that improve crop yield
- Cleaning products and disinfectants
- Water treatment chemicals
- Food preservatives and flavoring agents
Because of their wide use, amines are essential chemicals that support healthcare, agriculture, manufacturing, and environmental protection.
Definition of Amine in Chemistry
An amine is an organic compound that contains nitrogen bonded to carbon and hydrogen atoms. In chemistry, amines are formed when one or more hydrogen atoms of ammonia (NH₃) are replaced by carbon-containing groups. These carbon groups can be simple chains or aromatic rings.
In scientific terms, an amine is defined as a derivative of ammonia in which hydrogen atoms are substituted by alkyl or aryl groups. Because of this structure, amines show properties similar to ammonia but also have unique chemical behavior. Amines are widely studied in organic chemistry because they are present in many natural substances and industrial chemicals.
What Is an Amine Group?
An amine group is the functional group responsible for the chemical properties of amines. It consists of a nitrogen atom attached to one or more carbon atoms and hydrogen atoms. The general form of an amine group is –NH₂, –NHR, or –NR₂, depending on how many carbon groups are attached to the nitrogen.
This amine group is what gives amines their basic nature and high reactivity. It allows amines to take part in chemical reactions such as salt formation, bonding with acids, and synthesis of complex molecules. Many biological molecules, including amino acids and vitamins, contain an amine group.
Role of Nitrogen in Amines
Nitrogen plays a central role in amines. It has a lone pair of electrons, which makes amines chemically active. This lone pair allows amines to accept protons, which is why they behave as bases.
The presence of nitrogen also affects the shape, bonding, and reactivity of amines. Because nitrogen can form three bonds and hold a lone pair, it helps amines interact easily with other chemicals. This is the main reason amines are used so widely in medicines, agriculture, and industrial chemical processes.
Basic Structure of Amines
The amine structure is based on a nitrogen atom connected to carbon and hydrogen atoms. Amines are derived from ammonia, where one or more hydrogen atoms are replaced by carbon groups. The general formula of an amine can be written as R–NH₂, R₂–NH, or R₃–N, where R represents a carbon-containing group.
All amines contain at least one carbon–nitrogen (C–N) bond, which is the key feature of their structure. This bond decides how the amine behaves in chemical reactions and how it interacts with other compounds.
Role of Nitrogen and Lone Pair of Electrons
Nitrogen is the most important atom in an amine. It has five valence electrons, out of which three are used to form bonds with carbon or hydrogen. The remaining two electrons form a lone pair.
This lone pair is responsible for many important properties of amines. It allows amines to accept protons, take part in bonding, and react easily with acids. Because of this lone pair, amines act as bases and are widely used in chemical synthesis and industrial processes.
Shape and Bonding in Amines
Amines have a pyramidal shape around the nitrogen atom. This shape is due to the presence of the lone pair, which pushes the bonded atoms slightly downward. The bonding in amines is mainly single covalent bonding between nitrogen and carbon or hydrogen.
This shape affects how amines react and how strong their basic nature is. Smaller amines react faster, while larger amines show more steric effects.
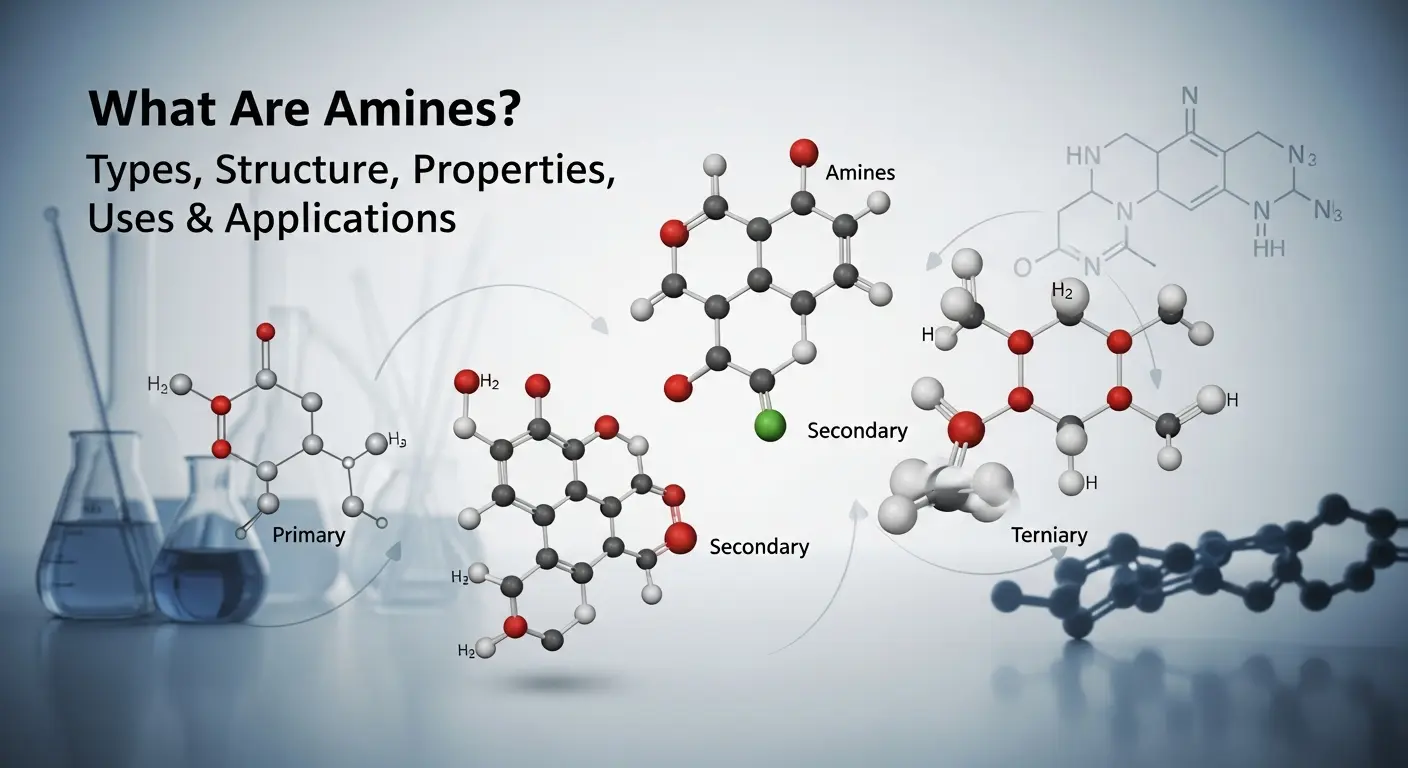
Structure of Primary Amines
A primary amine contains one carbon group and two hydrogen atoms attached to the nitrogen atom. Its general structure is R–NH₂. Primary amines are the simplest type of amines and are commonly used in laboratories and industries.
Difference Between Primary, Secondary, and Tertiary Amines
The structural difference between amines depends on the number of carbon groups attached to nitrogen:
- Primary amines (1°): One carbon group (R–NH₂)
- Secondary amines (2°): Two carbon groups (R₂–NH)
- Tertiary amines (3°): Three carbon groups (R₃–N)
As the number of carbon groups increases, the structure, reactivity, and basic strength of amines also change.
Main Types of Amines
Amines are classified into different types based on their structure and the nature of the carbon groups attached to the nitrogen atom. This classification helps in understanding their chemical behavior, reactivity, and industrial uses.
Classification Based on Number of Carbon Groups
This is the most common way to classify amines. It depends on how many carbon groups are bonded to the nitrogen atom.
Primary amines have one carbon group and two hydrogen atoms attached to nitrogen. Their general formula is R–NH₂. These amines are usually more reactive and are commonly used in the production of pharmaceuticals, dyes, and agrochemicals.
Secondary amines contain two carbon groups and one hydrogen atom attached to nitrogen. Their general structure is R₂–NH. Secondary amines are widely used as intermediates in chemical manufacturing and polymer production.
Tertiary amines have three carbon groups bonded to the nitrogen atom and no hydrogen atoms. Their general formula is R₃–N. Tertiary amines are less reactive toward some reactions but are very important in gas treatment, catalysts, and surfactant production.
Classification Based on Nature of Carbon Chain
Amines are also classified based on the type of carbon chain attached to the nitrogen atom.
Aliphatic amines have nitrogen attached to open-chain carbon groups such as alkyl groups. These amines are generally more basic and are commonly used in industrial processes, rubber chemicals, and water treatment applications.
Aromatic amines have nitrogen attached to an aromatic ring, usually a benzene ring. An example is aniline. These amines are less basic than aliphatic amines and are widely used in the manufacture of dyes, pigments, pharmaceuticals, and specialty chemicals.
This classification of amines helps chemists and industries choose the right type of amine for specific applications.
Common Examples of Amines
Amines exist in many forms and are found in natural substances, laboratory chemicals, and industrial products. Some amines are simple in structure, while others are complex and used in advanced applications.
Some widely known amines include:
- Methylamine (CH₃NH₂) – a simple amine used in chemical manufacturing
- Ethylamine (C₂H₅NH₂) – used in medicines and agricultural chemicals
- Aniline (C₆H₅NH₂) – an aromatic amine used in dyes and rubber processing
- Trimethylamine (N(CH₃)₃) – responsible for the fishy smell in spoiled fish
In nature, amino acids, which are the building blocks of proteins, also contain amine groups. This shows that amines are essential not only in industry but also in biological systems.
Example of a Primary Amine
A primary amine is an amine in which one carbon group and two hydrogen atoms are attached to the nitrogen atom. Its general formula is R–NH₂.
The most common example of a primary amine is methylamine. It is a colorless gas with a strong ammonia-like odor and is widely used as a starting material in chemical industries.
Another important primary amine is ethylamine, which is used in:
- Pharmaceutical manufacturing
- Pesticide and herbicide production
- Solvent and chemical synthesis
Aniline is a primary aromatic amine and plays a major role in:
- Dye and pigment production
- Rubber and plastic industries
- Pharmaceutical intermediates
Primary amines are highly reactive, which makes them valuable in many chemical processes.
Industrial and Laboratory Examples of Amines
In laboratories, amines such as diethylamine and triethylamine are commonly used as bases and reaction helpers in organic synthesis.
In industrial applications:
- Monoethanolamine (MEA) is used in oil and gas plants to remove carbon dioxide and hydrogen sulfide
- Dimethylamine is used in the production of solvents, surfactants, and rubber chemicals
- Triethanolamine is used in cosmetics, detergents, and cement additives
In the pharmaceutical industry, many drugs contain amine groups because they improve solubility and effectiveness.
This wide range of examples shows how amines are essential chemicals in research, manufacturing, healthcare, and daily-use products.
Are Amines Acidic or Basic in Nature?
Amines are basic compounds, not acidic. This means they can accept a proton (H⁺) in chemical reactions. The basic nature of amines comes from the presence of a nitrogen atom with a lone pair of electrons. Because of this, amines react easily with acids to form salts.
In most cases, amines show alkaline behavior when dissolved in water. Their solutions usually have a pH greater than 7, which clearly shows that amines are basic in nature.
Why Amines Show Basic Behavior
The main reason amines are basic is the lone pair of electrons on the nitrogen atom. This lone pair is free and ready to bond with a proton. When an amine reacts with an acid, the nitrogen donates its lone pair to accept the proton and forms an ammonium salt.
The strength of this basic behavior depends on how easily the lone pair is available. Factors such as attached carbon groups and molecular size can increase or decrease this availability.
Amines Compared to Ammonia
Amines are chemically related to ammonia (NH₃), but they are usually more basic than ammonia. This is because carbon groups attached to nitrogen push electrons toward the nitrogen atom. This increases the availability of the lone pair.
In simple terms:
- Ammonia has only hydrogen atoms attached to nitrogen
- Amines have carbon groups that increase electron density
Because of this, most aliphatic amines are stronger bases than ammonia. However, some aromatic amines can be weaker due to electron effects of the benzene ring.
How Structure Affects Basic Strength of Amines
The structure of an amine strongly affects how basic it is.
- Primary and secondary amines are usually stronger bases than tertiary amines in water
- Aliphatic amines are generally more basic than aromatic amines
- Larger carbon groups can sometimes reduce basicity due to steric effects
Solvent, temperature, and surrounding molecules also influence basic strength. Understanding this helps chemists choose the right amine for reactions, medicines, and industrial processes.
What Is Amine pKa?
Amine pKa is a value that shows the basic strength of an amine. In simple terms, it tells how easily an amine can accept a proton (H⁺). For amines, the pKa value usually refers to the conjugate acid of the amine, which is formed when the amine accepts a proton.
A higher pKa value means the amine is a stronger base, while a lower pKa value means it is a weaker base. Most common amines have pKa values between 9 and 11, though this can change depending on the structure of the amine.
Meaning of pKa in Amines
The pKa value helps chemists understand how stable the protonated amine is. When an amine has a high pKa, it holds onto the proton strongly. This means the amine is more basic and more reactive in acid–base reactions.
pKa is important in chemistry because it helps predict:
- Reaction behavior
- Solubility in water
- Salt formation
- Drug absorption in the body
- This is why pKa values are widely used in pharmaceutical and industrial chemistry.
Typical pH Range of Amines
When amines are dissolved in water, they usually form basic solutions. The typical amine pH range is between 8 and 11, depending on the type and concentration of the amine.
Simple aliphatic amines usually produce higher pH values, while aromatic amines show lower pH due to weaker basic nature. The pH value gives a quick idea about how alkaline an amine solution is.
How pKa Affects Amine Strength and Uses
The pKa value directly affects how an amine is used. Stronger amines (higher pKa) are preferred in neutralization reactions, gas treatment, and chemical synthesis. Weaker amines are often used in pharmaceuticals, where controlled reactivity is important.
In medicine, the pKa of an amine affects how a drug is absorbed, distributed, and excreted. This makes pKa a key factor in designing both industrial chemicals and medicines.
Physical and Chemical Properties of Amines
The properties of amines depend on their structure, size, and type. Both physical and chemical properties help in identifying amines and deciding their industrial and laboratory uses.
Physical Properties of Amines
Most amines are colorless compounds. Lower amines usually have a strong, unpleasant smell, often described as fishy or ammonia-like. As the size of the amine increases, the smell becomes less sharp.
Small amines are generally highly soluble in water because they can form hydrogen bonds with water molecules. As the carbon chain becomes longer, water solubility decreases, and the amines become more soluble in organic solvents.
The boiling point of amines increases with molecular size. Primary and secondary amines usually have higher boiling points than tertiary amines of similar size because they can form hydrogen bonds. Compared to alcohols, amines have slightly lower boiling points.
Chemical Behavior of Amines
Chemically, amines behave mainly as bases. They react easily with acids to form ammonium salts. This property is widely used in purification, drug formulation, and industrial processing.
Amines also take part in reactions such as:
- Alkylation and acylation
- Formation of amides
- Reactions with nitrous acid
Because of their nitrogen atom, amines act as good nucleophiles in many organic reactions.
Reactivity Trends in Amines
The reactivity of amines depends on their type and structure.
- Primary amines are generally more reactive than secondary and tertiary amines
- Aliphatic amines are more reactive than aromatic amines
- Bulky groups around nitrogen reduce reactivity
Understanding these trends helps chemists choose the correct amine for chemical synthesis, industrial production, and pharmaceutical development.
Industrial Production and Processing of Amines
Amines are produced on a large scale because they are essential for many industries, including pharmaceuticals, agriculture, dyes, and oil & gas. The production process depends on the type of amine required, whether aliphatic or aromatic. Industrial production is carried out in controlled conditions to ensure high purity, safety, and efficiency.
Raw Materials Used in Amine Production
The most important raw material for amine manufacturing is ammonia. Other raw materials include alcohols, alkyl halides, nitro compounds, nitriles, and hydrogen gas. Catalysts such as nickel, copper, or cobalt are added to speed up chemical reactions and improve product quality. The choice of raw materials depends on the type of amine desired and its industrial application.
Alkylation of Ammonia
One of the most widely used methods for producing amines is the alkylation of ammonia. In this process, ammonia reacts with alcohols or alkyl halides at high temperature and pressure in the presence of a catalyst. Depending on the ratio of ammonia to the reactants, the process can produce primary, secondary, or tertiary amines. The reaction mixture is then cooled, and the amines are separated and purified for industrial use. This method is highly efficient for producing aliphatic amines in bulk.
Reduction of Nitro Compounds
The reduction of nitro compounds is primarily used to produce aromatic amines, such as aniline. In this method, nitro compounds are reacted with hydrogen gas under the influence of a metal catalyst. The nitro group is converted into an amine group, resulting in high-purity amines. These amines are essential for pharmaceuticals, dyes, and chemical intermediates.
Reduction of Nitriles
Amines can also be produced by the reduction of nitriles, a process commonly used to make primary amines. In this reaction, nitriles react with hydrogen in the presence of catalysts. This method allows precise production of primary amines with minimal by-products and high efficiency.
Final Processing and Purification
After the chemical reactions, the product mixture is cooled, and unreacted raw materials are separated and recycled. The amines are then distilled and purified to meet industrial standards. Quality control checks are performed to ensure the amines are safe and suitable for use in pharmaceuticals, agriculture, and chemical manufacturing.
Key Industries That Use Amines
Amines are versatile chemicals used across many industries due to their reactivity, basic nature, and ability to form bonds with other compounds. Their applications range from manufacturing medicines to treating gases in oil refineries.
Pharmaceutical Industry
In the pharmaceutical industry, amines are essential for the synthesis of drugs. Many medicines, including painkillers, antibiotics, antihistamines, and antidepressants, contain amine groups. Amines help improve the solubility and stability of drugs, making them more effective. Pharmaceutical companies rely on high-purity amines as starting materials for chemical reactions and drug formulations.
Agriculture and Agrochemical Industry
Amines are widely used in agriculture as fertilizers, herbicides, and pesticides. They act as intermediates in producing nitrogen-based compounds that help enhance crop growth and protect plants from pests and diseases. Monoethanolamine (MEA) and other amines are commonly used in herbicide formulations. Their chemical properties make them ideal for efficient and stable agrochemical products.
Oil and Gas Industry
In oil and gas operations, amines are used for gas treatment and purification. They remove carbon dioxide (CO₂) and hydrogen sulfide (H₂S) from natural gas and refinery gases through chemical absorption. This process, called amine gas treating, ensures safer and cleaner fuels. Amines such as monoethanolamine and diethanolamine are the most commonly used chemicals in this industry.
Textile and Dye Industry
Aromatic and aliphatic amines are important in the textile and dye industry. They are used to produce dyes, pigments, and textile chemicals. Amines help create colorfast dyes that bond well with fabrics. Without amines, the production of high-quality textile dyes would not be possible.
Plastics, Rubber, and Polymer Industry
Amines are also used in plastics, rubber, and polymer manufacturing. They act as curing agents, catalysts, and intermediates in producing polymers, resins, and rubber chemicals. Their ability to react with acids and other compounds allows industries to produce materials with better strength, durability, and flexibility.
Water Treatment Industry
Amines also play a crucial role in water treatment. They are used to remove impurities, control pH, and neutralize acidic components in industrial water systems. Certain amines prevent corrosion in pipelines and boilers, making them essential for maintaining safe and efficient water management in factories and power plants.
Amines are therefore essential across multiple industries, making them one of the most versatile and widely used chemical compounds in modern manufacturing.
Applications and Uses of Amines
Amines are highly versatile chemicals with a wide range of applications in medicine, industry, daily life, and environmental processes. Their reactivity, basic nature, and nitrogen content make them essential in many chemical reactions and products.
Medical Uses of Amines
In medicine, amines are key components of many pharmaceutical drugs. They are found in antibiotics, painkillers, antihistamines, antidepressants, and vitamins. Amines help improve the solubility, stability, and bioavailability of drugs, making them more effective in the human body. Many synthetic and natural drugs rely on amine groups to interact with biological molecules and target specific diseases.
Industrial Uses of Amines
Industrially, amines are used as intermediate chemicals and additives. They are involved in the production of polymers, resins, rubber chemicals, dyes, and surfactants. Amines are also used in oil and gas treatment to remove acidic gases like CO₂ and H₂S, ensuring cleaner fuel and safer operations. The chemical reactivity of amines allows industries to manufacture complex products efficiently.
Daily Life Applications of Amines
Amines are present in many everyday products. They are used in cleaning agents, detergents, cosmetics, and food preservatives. Amines improve product stability and enhance performance. They are also found in flavorings and fragrances, showing how integral they are to common consumer goods.
Environmental Applications of Amines
Amines play an important role in environmental management. They are used in water treatment to neutralize acids, remove impurities, and prevent corrosion in pipes and boilers. Certain amines also help in waste treatment by binding harmful chemicals and making water safer for industrial and municipal use.
Because of these diverse applications, amines are essential chemicals that impact healthcare, industry, daily life, and environmental safety. Their versatility and effectiveness make them one of the most widely used chemical compounds globally.
Advantages and Limitations of Amines
Amines are widely used in chemistry, industry, medicine, and daily life due to their versatile chemical properties. However, like all chemicals, they have both benefits and limitations that must be understood for safe and effective use.
Key Benefits of Amines
Amines are highly valuable because of their reactivity, basic nature, and ability to form bonds with other compounds. They are essential in drug synthesis, agrochemicals, plastics, dyes, and water treatment. Their chemical structure allows them to participate in a wide range of reactions, making them flexible intermediates in industrial and laboratory processes. Amines also improve solubility and stability in pharmaceutical formulations and help create durable materials in polymers and rubber production. Their role in environmental applications, such as removing acidic gases from fuels and neutralizing impurities in water, demonstrates their practical significance.
Safety and Environmental Concerns
Despite their benefits, amines can pose safety and environmental risks if not handled properly. Many amines have a strong, unpleasant odor, and some can be toxic or irritating to skin and eyes. Release into the environment can cause water and air pollution, and improper disposal may harm aquatic life. Industrial operations using amines must follow strict handling protocols to prevent accidents, leaks, or exposure to workers.
Regulatory Considerations
The production, use, and disposal of amines are regulated by government authorities in most countries. Industries must comply with regulations related to chemical storage, transportation, emissions, and worker safety. For example, the Environmental Protection Agency (EPA) and similar agencies set limits on amine discharge and emissions. Regulatory compliance ensures that amines are used safely and sustainably while minimizing risks to humans and the environment.
In summary, amines are highly useful and versatile chemicals, but their safe handling, proper disposal, and adherence to regulations are essential to fully benefit from their advantages while minimizing risks.
Alternatives to Amines
While amines are widely used in industry, medicine, and daily life, there are situations where alternatives are preferred. Alternatives are needed to reduce toxicity, improve environmental safety, and comply with regulatory requirements. In some cases, amines can cause pollution, strong odors, or handling risks, making it important to explore safer and sustainable substitutes.
Why Alternatives Are Needed
Amines, especially in large-scale industrial use, can be toxic, irritating, or polluting. They can affect air quality, water systems, and worker health if not managed properly. Many industries are seeking alternatives that provide similar chemical functionality but with lower environmental and health risks. Using safer substitutes also helps companies meet green chemistry standards and regulatory compliance.
Amides as Alternatives
Amides are compounds similar to amines but have a carbonyl group attached to nitrogen. They are generally less reactive and less volatile than amines. Because of their stability and lower toxicity, amides are used in pharmaceuticals, polymers, and industrial chemicals as safer alternatives to some amines.
Alcohol-Based Compounds
Certain alcohol-based compounds can replace amines in chemical reactions and industrial processes. Alcohols are less toxic, easier to handle, and biodegradable, making them suitable substitutes in applications such as solvents, cleaning agents, and chemical intermediates. They are particularly useful where amines may react undesirably or pose safety risks.
Bio-Based and Green Chemistry Alternatives
With increasing focus on sustainability, bio-based and green chemistry alternatives are being developed. These include amino acids, plant-derived nitrogen compounds, and microbial amines. These alternatives are renewable, environmentally friendly, and often biodegradable, making them ideal for pharmaceuticals, agrochemicals, and water treatment.
In conclusion, while amines remain essential, industries are increasingly using amides, alcohol-based compounds, and bio-based alternatives to achieve safer, more sustainable, and environmentally responsible solutions.
Global Market of Amines
The amines market is growing steadily because of rising demand from key industries such as agriculture, pharmaceuticals, oil & gas, and industrial cleaning. In 2024, the global amines market was valued at over USD 21–22 billion and is expected to continue growing at a stable rate in the coming years. Analysts project that this market could reach between USD 30–35 billion by 2030, driven by increasing use in multiple industrial sectors worldwide.
Asia Pacific is the largest regional market for amines, accounting for a significant share of global demand. Countries like China, India, and Southeast Asian nations lead this growth due to rapid industrialization, expanding manufacturing bases, and strong demand from agriculture and chemical sectors. China remains the biggest producer and consumer, while India is rapidly expanding its capacity to serve both domestic and export markets.
Major Demand Drivers for Amines
Several factors drive the growth of the amine market. First, the agrochemical industry continues to grow, as more crop protection chemicals such as herbicides and pesticides are needed to support food production. Amines are key intermediates in making these products. Secondly, the pharmaceutical industry uses amines in a wide range of drugs and active ingredients, contributing to steady demand. Additionally, industries such as water treatment and gas purification rely on amines for removing impurities, boosting long-term use.
Consumer goods and personal care products also add to demand through the use of amine-based surfactants and conditioning agents. This broad range of applications helps the market remain resilient even when some sectors slow down.
Growing Industries Supporting Amines Demand
The pharmaceutical and healthcare sectors are rapidly expanding, particularly in emerging markets. Amines play an important role in developing medicines, antibiotic synthesis, and formulation of active compounds. The agrochemical sector continues to expand due to rising global food demand and the need for better crop yields. Water treatment and gas processing operations are also expanding, especially in developing economies, where infrastructure growth fuels demand for chemical treatment solutions.
Importance of Asia and India in the Amines Market
The Asia Pacific region remains the core amines market hub due to fast industrialization, lower production costs, and strong manufacturing networks. China dominates global consumption, while India is rapidly increasing its production capacity, driven by domestic demand and export opportunities. India’s growth in pharmaceuticals and agriculture contributes significantly to its rising need for amines.
Overall, the amines market continues to grow worldwide, supported by multiple expanding industries and robust demand in Asia, especially India and China. This makes amines one of the most important and widely used chemical segments in the global economy.
Safety and Environmental Impact of Amines
Amines are widely used chemicals, but their handling and disposal require careful attention because they can pose health and environmental risks. Understanding these risks is essential for safe industrial, laboratory, and commercial use.
Safety Concerns
Many amines, especially low molecular weight aliphatic amines, have a strong, unpleasant odor and can be irritating to the skin, eyes, and respiratory system. Prolonged or high exposure may cause respiratory distress, skin burns, or eye damage. Workers handling amines in industrial settings must use protective equipment, such as gloves, goggles, and proper ventilation, to minimize exposure.
Some amines are also flammable and can form explosive mixtures with air under certain conditions. Therefore, storage and transportation of amines require controlled temperature, proper containers, and fire safety measures.
Environmental Impact
Amines released into the environment can affect air, water, and soil quality. In water systems, high concentrations of amines can increase pH levels, react with other chemicals, and harm aquatic life. In the air, volatile amines can contribute to odorous emissions and chemical pollution. Improper disposal of industrial amine waste can lead to long-term environmental contamination.
Mitigation Measures
To reduce risks, industries follow strict handling and disposal protocols. Waste amine solutions are often neutralized with acids, treated biologically, or recovered for reuse. Proper ventilation, spill containment, and regular monitoring are critical to ensure workplace safety and environmental protection. Regulatory agencies such as the EPA in the USA and similar bodies worldwide enforce limits on amine emissions and effluents to minimize environmental impact.
In conclusion, while amines are highly useful and versatile, their safe use requires attention to health, safety, and environmental considerations. Proper handling, storage, and waste management ensure that the benefits of amines can be enjoyed without causing harm to humans or the environment.
Conclusion
Amines are fundamental chemicals with wide-ranging applications in pharmaceuticals, agriculture, industrial processes, water treatment, and daily life products. Their unique chemical structure, featuring a nitrogen atom with a lone pair, gives them basic properties, reactivity, and versatility, making them essential for modern chemical manufacturing.
They are classified into primary, secondary, and tertiary amines, and can be aliphatic or aromatic, each type serving specific purposes in industries. Amines are used in drug synthesis, dyes, polymers, gas treatment, and environmental management, highlighting their importance in both industrial and everyday contexts.
While amines offer significant benefits, such as high reactivity, chemical flexibility, and industrial utility, they also require careful handling due to safety and environmental concerns. Regulatory compliance, proper storage, and controlled disposal are necessary to minimize health risks and environmental impact.
The global market for amines continues to grow, driven by rising demand in Asia, particularly India and China, along with expanding pharmaceutical, agrochemical, and industrial sectors. Industries are also exploring alternatives like amides, alcohol-based compounds, and bio-based chemicals to reduce environmental and safety risks.
In summary, amines remain one of the most versatile and widely used chemical compounds, playing a crucial role in modern chemistry, industry, and daily life. Proper understanding of their properties, applications, and safety measures ensures they can be utilized effectively and sustainably, benefiting both industry and society.
FAQs –
1. What are amines?
Amines are organic compounds that contain a nitrogen atom bonded to hydrogen and/or carbon atoms. They are derived from ammonia and are widely used in industry and daily life.
2. What is an amine group?
An amine group is the -NH₂, -NHR, or -NR₂ part of a molecule that contains nitrogen. It is responsible for the basic nature and chemical reactivity of amines.
3. Are amines acidic or basic?
Amines are basic because the nitrogen atom can accept a proton (H⁺). Their basicity depends on the type and structure of the amine.
4. What are the types of amines?
Amines are classified into primary (1°), secondary (2°), and tertiary (3°) amines based on the number of carbon groups attached to nitrogen. They can also be aliphatic (open chain) or aromatic (attached to a benzene ring).
5. What is a primary amine?
A primary amine has one carbon group attached to nitrogen and two hydrogen atoms. Example: methylamine (CH₃NH₂).
6. What are common uses of amines?
Amines are used in medicine (drugs), agriculture (fertilizers and pesticides), industry (polymers, dyes, and gas treatment), daily life products (detergents and cosmetics), and water treatment.
7. How are amines produced?
Amines are made using ammonia and other chemicals. Common methods include alkylation of ammonia, reduction of nitro compounds, and reduction of nitriles.
8. What are the environmental risks of amines?
Amines can be toxic, irritating, or polluting if released into air or water. Proper handling, storage, and disposal are necessary to protect workers and the environment.
9. Are there alternatives to amines?
Yes, industries sometimes use amides, alcohol-based compounds, and bio-based or green chemicals to reduce toxicity and environmental impact.
10. Which industries use amines the most?
Amines are widely used in pharmaceuticals, agriculture, oil & gas, textiles and dyes, plastics and polymers, and water treatment.